Growing demand for lentiviral vectors (LVVs) is creating the need for robust, scalable, GMP manufacturing processes. Accelerating process development can be difficult, however, due to a lack of rapid, reliable methods for LVV quantification. The Virus Counter® Plus platform (Sartorius) using the Virotag® VSVG assay enables quick (near-real-time) and precise determination of total particle counts and therefore supports rapid development of high-performing processes.
In this 3-part blog series, scientists from ABL, Inc. discuss the issues with current titer measurement methods, describe the Virus Counter Plus platform, and present highlights of a recent study conducted in collaboration with Sartorius to assess the correlation of titer data obtained using the new method to that generated using traditional physical and functional titer assays.
A growing demand for lentiviral vectors
Lentiviral vectors (LVVs) have largely been used to deliver genetic material used for ex-vivo cell therapy production. Advances in LVV engineering are now also driving greater interest in LVVs as vectors for in vivo gene delivery. The rising demand for LVVs is creating a need for more robust, scalable, GMP manufacturing processes.
ABL is a CMO with a history of producing and purifying biomolecules for applications including oncolytics, vaccines, and cell and gene therapies. ABL has over forty years of experience in HIV research and development, including development of the first HIV diagnostic blood test. Lentivirus is derived from HIV and as such, ABL is uniquely positioned to utilize its HIV expertise to establish a lentivirus purification process.
The use of lentivirus in cell and gene therapies has expanded over the last few years, especially after the FDA approval of several CAR-T cell therapies for diseases such as β-thalassemia and cerebral adrenoleukodystrophy (CALD). The positive outcomes of these treatments have prompted the need for more lentivirus production and purification. Lentivirus has several unique properties that make it a suitable vector for cell therapy, such as:
- Large genetic capacity- ability to carry genes up to 8 kb.
- Ability to integrate into the host genome which allows for long-term expression of transfected genes.
- Ability to infect different kinds of cell types, including dividing and non-dividing cells.
- Reduced capacity to elicit an immune response once infected in a host.
The challenges of working with lentiviral vectors
Growing interest in lentiviral vectors necessitates the development of a LVV purification process that can be easily scaled to manufacturing level. However, lentivirus is a challenging virus to work with. Some reasons are outlined below:
- It is a fragile vector and sensitive to many external factors such as pH, shear force, salt concentration and temperature changes. As a result, the purification process must be rapid and efficient while maintaining gentle conditions to restrict loss of infectivity. Otherwise, low functional titer recovery and a high viral particle to functional titer ratio (VP/TU ratio) would be observed. An upwards trend in the ratio indicates the generation of partial or damaged viral particles during the purification process.
- Sterility is a pre-requisite for viral vectors to ensure safety, typically achieved through aseptic processing or sterile filtration. When high titers are achieved during the purification process, the sterile filtration of such a high titer virus at the end of the process is a major challenge and results in low recovery. In addition, a high concentration of impurities causes the lentivirus to aggregate in the final concentration step, making sterile filtration a challenge at this stage.
- Starting with suspension cell culture offers higher viral productivity than an adherent system but also results in high levels of residuals such as host cell protein (HCP), host cell DNA (hcDNA) and cell debris. Removal of these impurities by the end of the process is necessary to ensure product safety and to meet regulatory requirements.
Establishing robust LVV manufacturing processes
ABL attempted to address these challenges in LVV purification and establish a purification platform by employing a methodical, step-by-step approach. First, the effect of potential critical process parameters at each step on lentiviral recovery and quality was assessed and optimized at small scale. The refined process parameters were then used in scale-up runs to purify the viral vector, where a high functional titer of final product and satisfactory reduction of both HCP and hcDNA levels were achieved. Figure 1 describes the downstream purification strategy we developed after testing several parameters at each step during numerous trials.
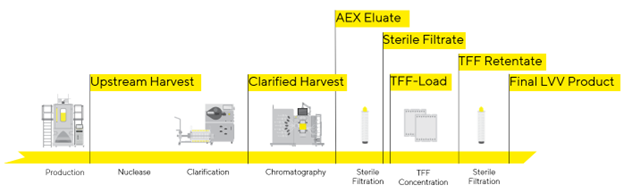
Figure 1: Flow diagram of Lentivirus Purification
One essential component of LVV purification is the analytical assays performed to determine physical and functional titer. LVV is subject to degradation under a host of external conditions (see above) and generally, the quicker the analytical assays can be run, the faster that the LVV can be applied to the patient.
Traditional methods for physical titer determination, including quantitative polymerase chain reaction (qPCR) and p24 enzyme-linked immunosorbent assay (ELISA) techniques, provide results in 1-2 days but do not directly count viral particles and typically are labor-intensive. ABL utilized the Lenti-X™ qRT-PCR Titration Kit (from TakaraBio) and the p24 ELISA (from ABL, Inc.) to analyze titer at each step of the process. The qPCR analyses were performed in triplicate, with viral genomes/mL results converted to vp/mL. Initial p24 concentration values were multiplied by 10,000,000 (1×10⁷) (conversion factor from Didier Trono) to determine the vp/mL.
In addition to physical titer assays, the functional titer was measured for each step of the purification process. While functional titers provide a direct measurement of the number of infectious viral particles, determination of functional titers involves cell-based transduction assays that can be highly variable and take 2-4 weeks to complete. TU assays were performed by transducing HT1080 cells with virus at three dilutions and quantifying the GFP-positive signal using a fluorescence-activated cell sorter (FACS).
While traditional quantitation methods provide important information on the virus titer that is used to determine dosage, the assays themselves are very laborious and can be time consuming, limiting quick turnaround of LVV production. Also, an essential component of the purification process is the final concentration of purified LVV. Typically, a dosage of 1E+08 TU/mL of virus is administered to the patient. Prior to the TFF step, it is advantageous to know the virus titer to calculate the fold of concentration needed during TFF. Current methods to determine titer do not provide quick results that would be useful at this step.
The ability to eliminate the need for lengthy and variable analytical techniques and achieve quicker, more robust titer data acquisition with less hands-on time is critical to support accelerated development of robust LVV manufacturing processes. Part Two of this series will focus on the Virus Counter Plus from Sartorius and its utility in obtaining quick and accurate titer results.
Authors: Poorni Adikaram, PhD, Tyler Frazier and Timothy Fouts, PhD, CSO at ABL Inc. and Rebecca Montange, PhD and Brandon Harrell at Sartorius
Learn more about the Virus Counter Plus instrument from Sartorius in Part Two, or find the results of a recent study assessing the correlation of titer data obtained using new and standard analytical methods in Part Three.
Would you like to discuss how Ascend ABL can support your LVV production? Contact us using the form below.


